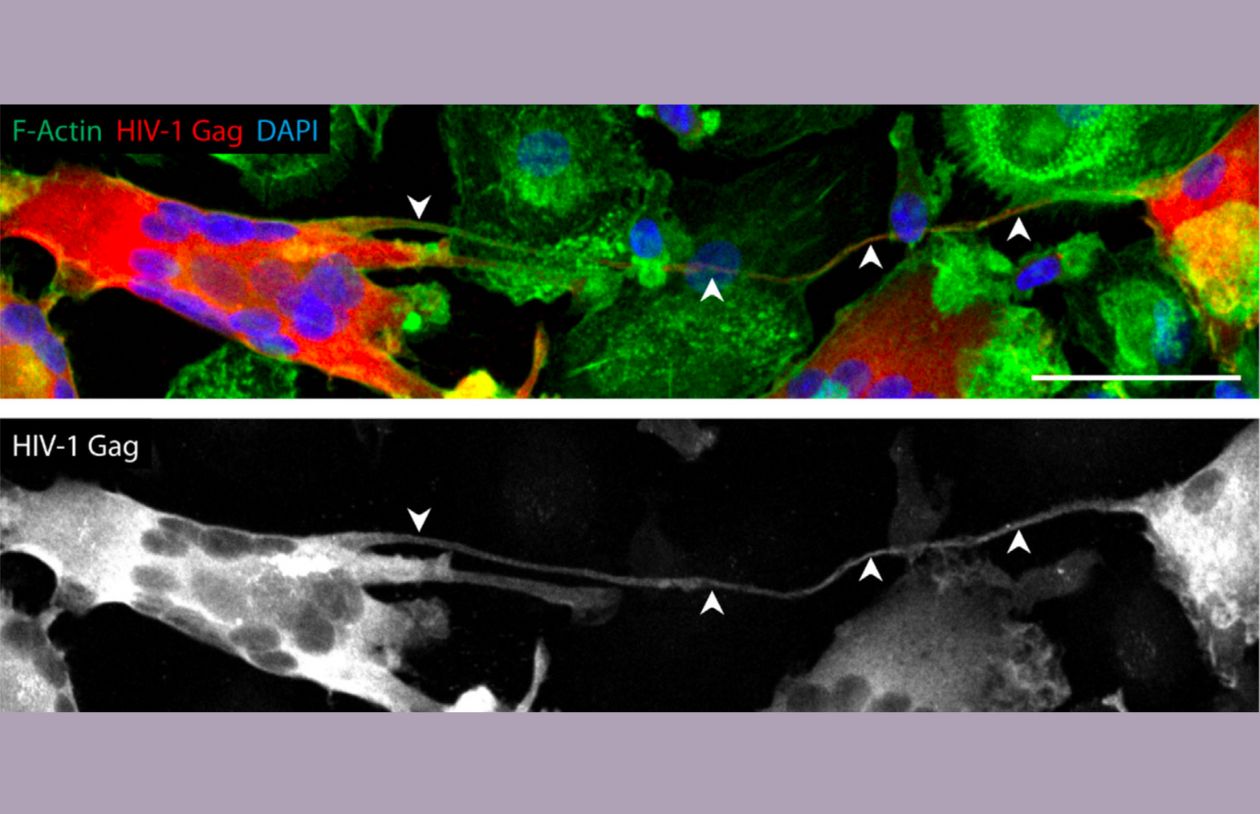
Images of tunneling nanotubes. Arrows pointing to the tunneling nanotubes. Credit: Dupont M., Tunneling nanotubes in immune cells, CC BY 4.0, Confocal image of day 13 HIV-1-infected human monocyte-derived macrophages and MGC interconnected through a TNT. Arrowheads show a TNT. HIV-1 Gag (red), F-actin (green), DAPI (blue). Scale bar, 50 µm. via Wikimedia Commons
From his lab at the University of Houston College of Pharmacy, Mingfu Wu, associate professor and member of the Drug Discovery Institute, is offering new hope for treating heart disease by sharing insights into the fundamental process of how the heart is formed in utero. His findings, published in Science, focus on long, thin, channels of membranes – called Tunneling Nanotube-Like Structures - that connect cells together. Wu reports the structures may be a fundamental way for cells to communicate, offering new insights into heart disease development and potential treatments.
The formation of the heart depends on critical signals exchanged between its two main layers: the myocardium (the muscle that powers the heartbeat) and the endocardium (the inner lining of the heart). This communication is essential for ensuring the heart takes shape correctly and functions effectively.
“Here we characterized Tunneling Nanotube-Like Structures (TNTLs), which we found physically connecting cardiomyocytes in myocardium to endocardial cells in endocardium,” reports Wu.
“These structures likely help to facilitate long-distance intercellular communication essential for heart formation.”
Lianjie Miao, research assistant professor of pharmacology, is the paper’s first author.
The signals are particularly important during development of the trabeculae, which are crucial in early heart development. Before the coronary system develops, trabeculae supply blood by increasing the inner surface area of the heart wall and enhancing the exchange of oxygen and nutrients between blood and the heart wall.
Wu and the team used genetic labeling, contact tracing techniques and advanced imaging to demonstrate the existence of the tunneling nanotubes in embryonic hearts. They found that the tubes extended across the heart’s main layers and cardiac jelly, establishing direct connections that enable cells to communicate and transfer proteins that carry out important functions.

“We found that TNTLs were able to transport signaling molecules, cytoplasmic proteins, and trafficking vesicles, underscoring their role as conduits for intercellular communication and proving them essential in cardiac morphogenesis,” said Wu.
“Disruption of TNTLs in embryonic hearts resulted in impaired ventricular wall morphogenesis, evidenced by loss of trabeculae and defective myocardial growth,” he said.
Wu said future research should explore the molecular machinery governing TNTLs formation, the potential functions of protein transfer between myocardium and endocardium during heart formation and how adjusting or controlling these structures could lead to new ways to treat congenital heart defects and heart failure.